Blogtext
Process Monitoring in the Pharmaceutical Industry - The Latest Publication on b.sure®
Nothing is more important in the production of pharmaceuticals than a flawless and smooth workflow. Due to the networking of processes and applications, digitalisation is becoming increasingly important in the pharmaceutical industry. But it is precisely the desired, all-encompassing monitoring and an optimal production process that present manufacturers, users and, of course, suppliers with considerable challenges. Even more so because a lot of processes still rely on analogous solutions.
Practical experience shows that due to increasing automation and interlinking of production processes, fewer and fewer employees have to monitor complete production lines. Moving back and forth between the individual operating and control panels of the machines puts physical strain on the operators.
Plant operators must, however, have access to the operating panels at all times in order to detect deviations from process parameters or error messages at an early stage. This is not always possible if the plants are located in different rooms. To date, fault and warning signals have often been displayed only by means of status indicators. However, if the machine operator does not have a direct or permanent view of the status light, the plant may stop undetected in the event of a fault or continue to run with incorrect parameters and produce a faulty batch.
Constant information about the production status
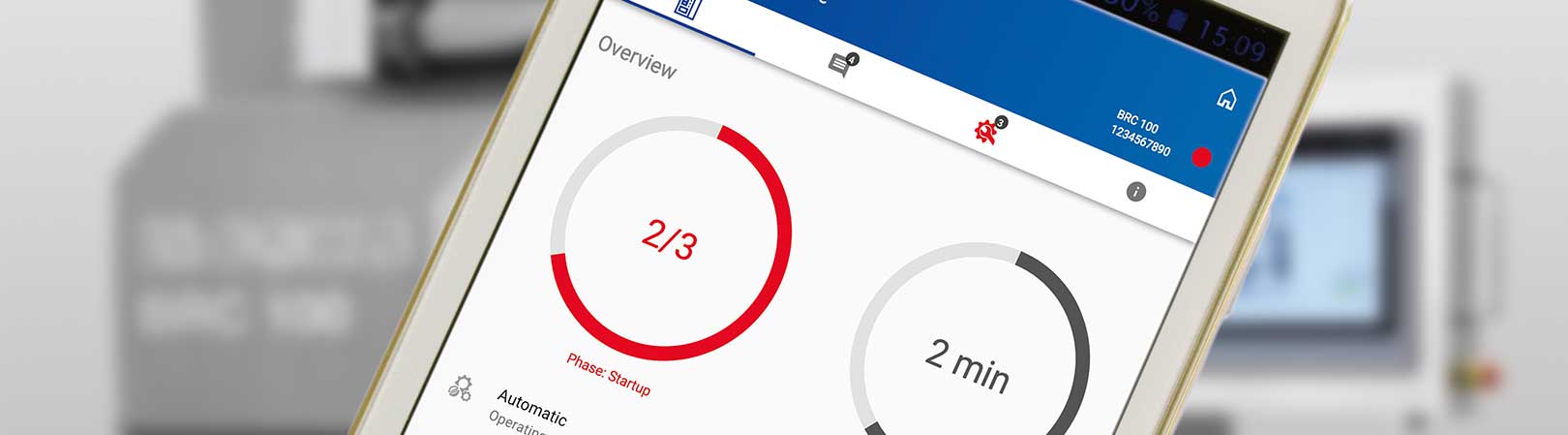
L.B. Bohle Maschinen und Verfahren, headquartered in Ennigerloh, Germany, solves this issue with its new process monitoring application b.sure. The b.sure® app avoids downtime or faulty production by sending operating data and parts of the process data in real time to a communications server and processing them.
All systems with programmable logic controllers (PLCs) already collect a wide range of process and operating data which is immediately available on the machines. This means that the customer or system operator can now keep track of the processes from authorized end devices or at the PC workstation. The application informs the user of:
- Machine name with serial number,
- Operating status (process active),
- Remaining process time,
- Batch data, batch number and recipe,
- Process values (e.g. force, gap width and throughput),
- Errors and status messages (e.g. emergency stop or if ACTUAL-values leave the permitted predefined range) and
d - Maintenance mode with notification function.
In contrast to other process monitoring solutions on the market with an external cloud solution, L.B. Bohle takes the particularities of the highly-sensitive pharmaceutical environment into account with b.sure® and processes data in the customer’s network. This means that the data cannot be communicated outside the company and cannot be stored permanently.
Following analysis of the available parameters, L.B. Bohle has developed a communications server that can be easily integrated into the existing IT infrastructure of the respective company. The data is then processed on this internal platform for further processing via app or email. Thus, the user receives current status messages via mobile devices and throughout the entire company, regardless of location.
The application can be flexibly configurated, and the user can individually determine which information is recorded by which system and in which form it is to be evaluated. In a further step, it is defined which signals or warnings (acoustic and optical warning in case of malfunction / alarm) with which content are forwarded to whom. With b.sure®, both data and process security are given absolute priority.
Authorized operators pursue the process
In the event of a malfunction/error, the corresponding message is sent to the end devices via an email service. The app is web-based and can be opened on the respective end device or by email. The user is, therefore, not bound to a workstation.
The system operator is not only informed by message in the event of a system fault, but also when the parameters are outside the set values. This has the advantage that the operator can also monitor several systems in parallel and react quickly. It saves valuable time in the event of a malfunction in order to guarantee the quality of the product.
b.sure is particularly safe because systems and authorized operators must be defined in advance by an administrator on the communications server. This prevents unauthorized access. One-way communication provides further protection against incorrect interference with the process control, as the user has no possibility whatsoever to directly influence or control the process via the end devices.
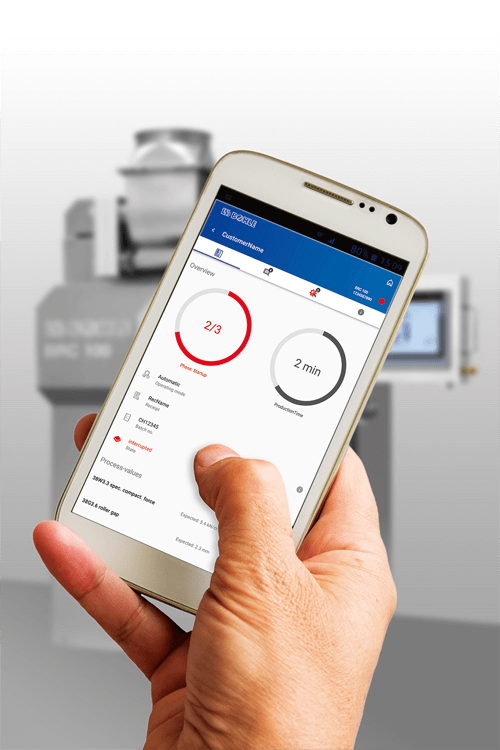
Constant updates on maintenance intervals
In addition, the b.sure® application contains a maintenance mode with notification function, in which information about impending maintenance and calibration is transmitted. This ensures that the system is always up to date. It also simplifies maintenance planning and traceability.
Ansprechpartner
Your contact for b.sure ®
Burkhard Schmidt
Verkaufsleiter
inquiry@lbbohle.de
+49 2524 – 93 23 0



